Patient-Centric Innovations, Decentralized Protocols, and Digital Consent Platforms Defining UK Clinical Trials Market Trends
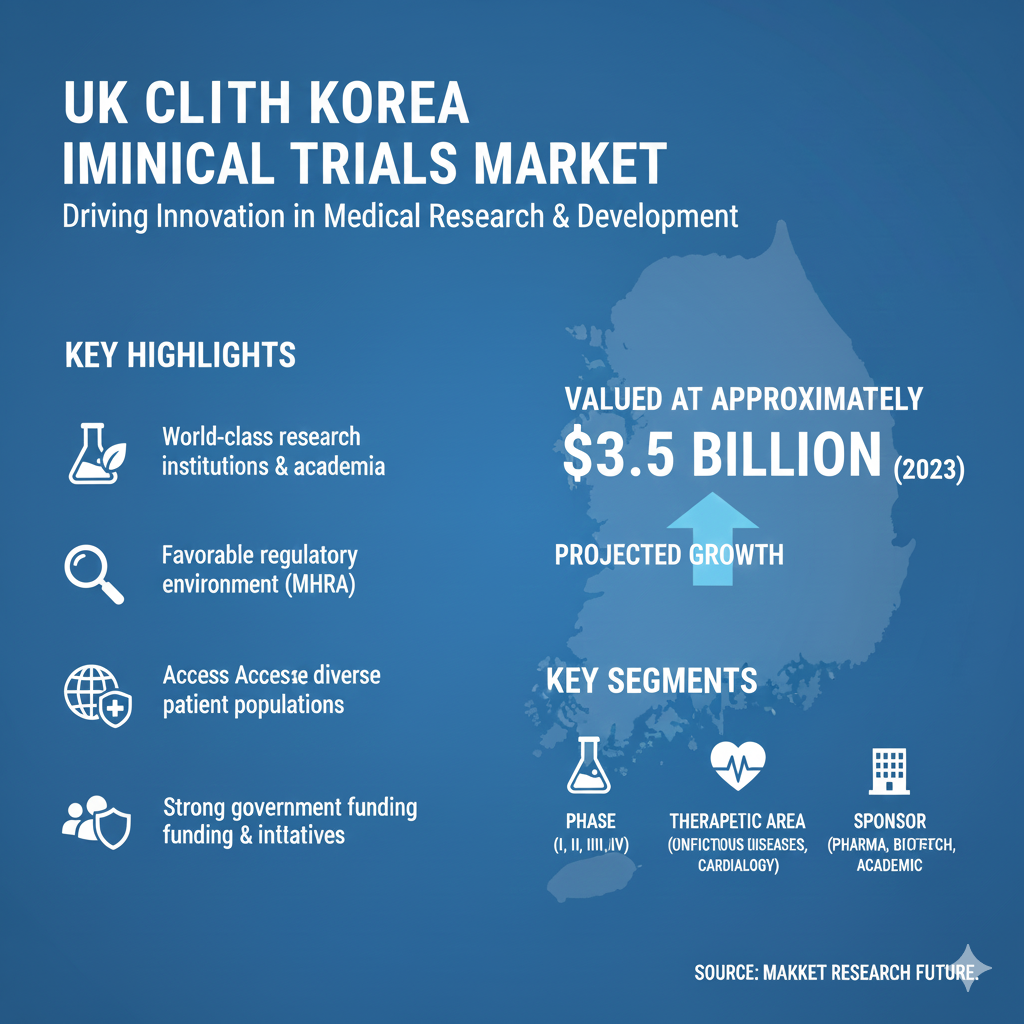
The UK Clinical Trials Market is shifting toward patient-centric trial designs that integrate decentralized care pathways and digital consent systems. Remote onboarding, electronic documentation, and telemedicine-driven consultations are helping eliminate logistical barriers associated with traditional site-based trials. Pharmaceutical stakeholders are prioritizing solutions that improve patient retention, shaping emerging patterns seen in UK Clinical Trials Market Trends reports addressing modernized participation models.
Digital consent platforms reduce administrative complexity by enabling secure document verification and remote identity authentication. These systems ensure compliance while simplifying the enrollment journey. Remote clinical oversight supported by mobile diagnostics allows patients to perform certain trial procedures at home, thereby improving convenience and increasing trial inclusivity among diverse populations.
Decentralized monitoring tools also enable remote data integration, allowing investigators to receive continuous updates from wearable devices, biosensors, or digital patient diaries. These capabilities allow trial designers to collect real-world, high-frequency data without requiring long-term on-site visits. By simplifying engagement, the UK is developing an accessible and high-tech trial environment.
FAQs
Q1. How do digital consent systems improve patient experience?
They simplify enrollment and allow secure remote approval.
Q2. Why are decentralized protocols rising in popularity?
They improve access, reduce travel burden, and enhance participant diversity.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



