Cell and Gene Therapy Market : Driving the Future of Personalized Medicine
Cell and Gene Therapy Market Overview
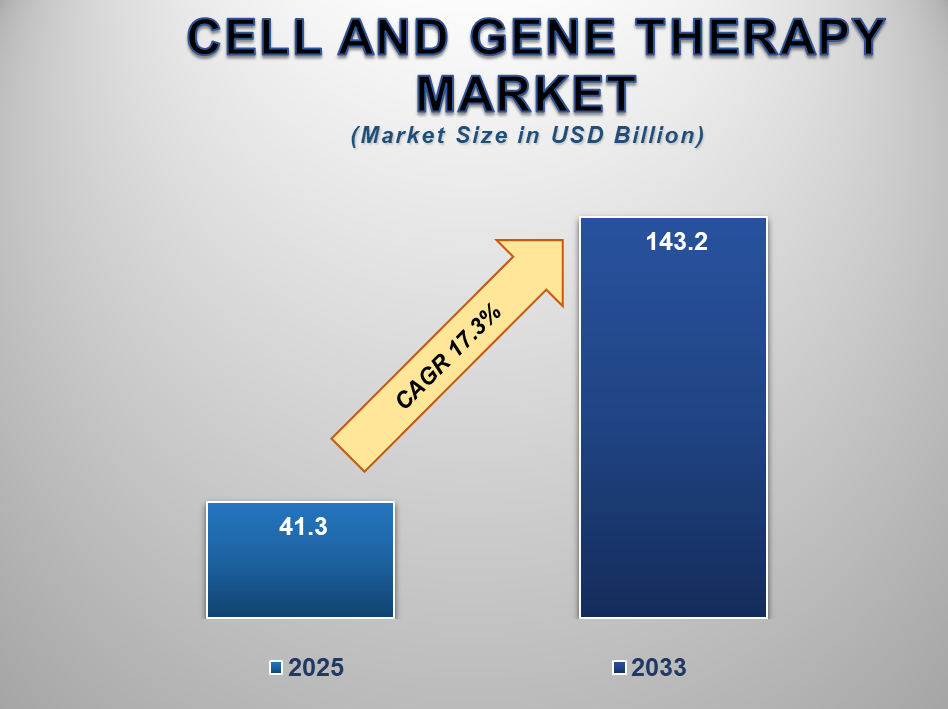
The Global Cell and Gene Therapy Market is witnessing rapid expansion, driven by breakthroughs in regenerative medicine, rising prevalence of chronic and genetic disorders, and increasing investments in advanced therapeutic research. Valued at USD 41.3 billion in 2025, the market is projected to reach USD 143.2 billion by 2033, growing at a remarkable CAGR of 17.3% during the forecast period.
Get Report Link : https://m2squareconsultancy.com/reports/cell-and-gene-therapy-market
Understanding Cell and Gene Therapy
Cell therapy involves the administration of live, intact cells into a patient to repair or replace damaged tissue or cells. Examples include stem cell therapy and chimeric antigen receptor (CAR) T-cell therapy for cancer treatment. Gene therapy, on the other hand, focuses on the modification or replacement of faulty genes within a patient’s cells to treat or prevent diseases. These therapies can be administered in vivo (inside the body) or ex vivo (outside the body, before being reintroduced).
When combined, cell and gene therapy represents a frontier of medicine capable of transforming healthcare by offering personalized, long-lasting, and potentially curative treatments.
Sample Report Link : https://m2squareconsultancy.com/request-sample/cell-and-gene-therapy-market
Buy Now https://m2squareconsultancy.com/purchase/280
Market Growth Drivers
-
Rising Prevalence of Chronic and Genetic Disorders
The growing incidence of cancers, rare genetic conditions, and autoimmune diseases has intensified the need for advanced therapeutic options. According to health organizations, more than 350 million people worldwide suffer from rare diseases, many of which are genetic in nature and can benefit from CGT. -
Advancements in Biotechnology and Genomics
Innovations such as CRISPR-Cas9 genome editing, next-generation sequencing (NGS), and improved viral and non-viral vectors have revolutionized the efficiency and safety of gene therapies. -
Regulatory Approvals and Supportive Policies
Agencies like the U.S. FDA and the European Medicines Agency (EMA) have introduced expedited approval pathways such as Breakthrough Therapy Designation and Orphan Drug Designation, enabling faster market access for cell and gene therapies targeting life-threatening diseases. -
Strong Investment and Collaborations
Venture capital firms, pharmaceutical giants, and biotech startups are pouring billions into CGT research. Strategic collaborations between academic institutions and industry players have accelerated clinical trials and commercialization. -
Growing Acceptance of Personalized Medicine
Patients and healthcare providers are increasingly embracing therapies tailored to individual genetic profiles, fueling demand for CGT solutions.
Market Challenges
Despite its promise, the cell and gene therapy market faces significant hurdles:
-
High Costs of Therapy: Treatments like CAR-T therapies can cost upwards of $400,000 to $1 million per patient, limiting accessibility.
-
Complex Manufacturing and Logistics: CGT production often involves customized, small-batch manufacturing, requiring stringent quality control and advanced infrastructure.
-
Regulatory and Ethical Concerns: Gene editing, particularly germline modification, raises ethical debates, while global regulatory landscapes remain fragmented.
-
Long-Term Efficacy and Safety: Since CGT is relatively new, there is limited long-term data on efficacy, durability, and potential side effects.
Market Segmentation
-
By Therapy Type:
-
Cell Therapy: Stem cell therapy, CAR-T therapy, dendritic cell therapy.
-
Gene Therapy: Gene editing, gene silencing, viral and non-viral vector therapies.
-
-
By Application:
-
Oncology (largest segment, driven by CAR-T therapies)
-
Rare genetic disorders (e.g., spinal muscular atrophy, hemophilia)
-
Neurological diseases (Parkinson’s, Alzheimer’s research)
-
Cardiovascular and ophthalmic disorders
-
-
By Region:
-
North America dominates due to advanced healthcare infrastructure, high R&D investment, and supportive regulations.
-
Europe follows with strong adoption in oncology and rare diseases.
-
Asia-Pacific is expected to witness the fastest growth, supported by expanding clinical trials in China, Japan, and South Korea.
-
Key Market Players
Several leading companies are shaping the competitive landscape:
-
Novartis AG – Known for Kymriah, the first FDA-approved CAR-T therapy.
-
Gilead Sciences (Kite Pharma) – Developer of Yescarta, another leading CAR-T therapy.
-
Bluebird Bio – Focused on genetic therapies for rare diseases.
-
Bristol Myers Squibb – Advancing multiple CAR-T and gene therapy pipelines.
-
Sangamo Therapeutics, Spark Therapeutics, and CRISPR Therapeutics – Innovating in gene editing and rare disease treatment.
Future Outlook
The global cell and gene therapy market is projected to grow at a double-digit CAGR over the next decade, with revenues potentially reaching hundreds of billions by 2035. The ongoing research in CRISPR-based therapies, allogeneic “off-the-shelf” cell therapies, and next-gen viral vectors will enhance scalability and affordability. Additionally, increased partnerships between biotech firms, governments, and healthcare providers will play a pivotal role in expanding patient access.
In the long term, CGT is expected to move beyond oncology and rare diseases into broader applications such as cardiovascular repair, diabetes, and regenerative medicine. With technological advances, streamlined regulatory pathways, and cost-optimization strategies, cell and gene therapy may become a cornerstone of modern medicine.
Conclusion
The cell and gene therapy market represents one of the most promising frontiers in healthcare, offering hope for cures rather than symptomatic treatments. Although challenges such as high costs and complex manufacturing remain, the continuous wave of innovation, regulatory support, and rising demand for personalized medicine will drive strong growth. As more therapies gain approval and reach commercial scale, cell and gene therapies are poised to redefine the future of medicine, transforming patient care and improving global health outcomes.
Buy Now https://m2squareconsultancy.com/purchase/280
About m2squareconsultancy :
We are a purpose-driven market research and consulting company passionate about turning data into direction. Founded in 2023, we bring together researchers, strategists, and data scientists who believe that intelligence isn’t just about numbers, it’s about insight that sparks progress.
We cater to a wide range of industries by delivering customized solutions, strategic insights, and innovative support that help organizations grow, adapt, and lead in their respective sectors. Here’s a brief overview of key industries we work with
Contact Us:
Email: sales@m2squareconsultancy.com
Phone (IN): +91 80978 74280
Phone (US): +1 929 447 0100
More Report Links :
https://m2squareconsultancy.com/reports/probiotic-drinks-market
https://m2squareconsultancy.com/reports/self-adhesive-labels-market
https://m2squareconsultancy.com/reports/rare-disease-drugs-market
https://m2squareconsultancy.com/reports/sickle-cell-anemia-testing-and-screening-market
https://m2squareconsultancy.com/reports/urine-testing-cups-market
https://m2squareconsultancy.com/reports/global-vaccines-market
https://m2squareconsultancy.com/reports/surgical-instruments-market
https://m2squareconsultancy.com/reports/predictive-analytics-market
https://m2squareconsultancy.com/reports/smart-cities-market
https://m2squareconsultancy.com/reports/seed-treatment-market
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jeux
- Gardening
- Health
- Domicile
- Literature
- Music
- Networking
- Autre
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



