Platelet Rich Plasma Market Report: Unlocking Growth Potential and Addressing Challenges
United States of America – [October 27, 2025] – The Insight Partners is proud to announce its latest report, “Platelet Rich Plasma Market: An In-Depth Analysis of the Global Industry Landscape.” The report provides a comprehensive view of the Platelet Rich Plasma (PRP) Market, describing the current scenario, emerging trends, and growth estimates during the forecast period 2023–2031.
Overview of the Platelet Rich Plasma Market
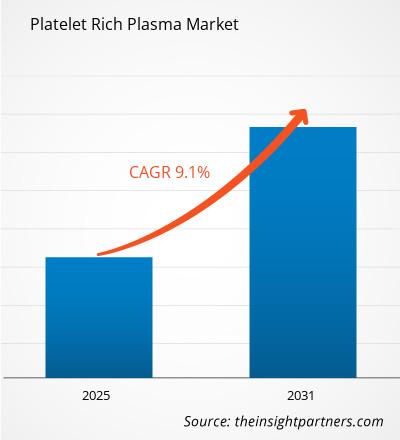
The Platelet Rich Plasma Market is witnessing dynamic expansion, fueled by rising applications in orthopedics, sports medicine, aesthetics, and wound healing. PRP therapy — derived from autologous blood concentrates enriched with platelets — is increasingly recognized for its regenerative potential. Its ability to accelerate tissue repair and reduce inflammation is transforming treatment protocols across both medical and cosmetic sectors.
This report provides in-depth insight into the market drivers such as technological innovations in PRP preparation systems, growing patient awareness, regulatory evolution, and an increasing preference for non-surgical treatment alternatives.
Market Segmentation
Segmentation Criteria:
1. By Type
o Pure Platelet Rich Plasma (P-PRP)
o Leukocyte-Rich Platelet Rich Plasma (L-PRP)
o Pure Platelet-Rich Fibrin (P-PRF)
o Leukocyte-Rich Platelet-Rich Fibrin (L-PRF)
2. By Origin
o Autologous PRP
o Allogeneic PRP
o Homologous PRP
3. By Application
o Orthopedics and Sports Medicine
o Dermatology and Aesthetics
o Neurosurgery
o Dentistry
o Wound Healing
o Others (Ophthalmology, Cardiology)
4. By End-User
o Hospitals and Clinics
o Research and Academic Institutions
o Ambulatory Surgical Centers
5. By Geography
o North America: United States, Canada, Mexico
o Europe: Germany, UK, France, Italy, Spain, Rest of Europe
o Asia-Pacific: China, Japan, India, South Korea, Australia, Rest of APAC
o Middle East & Africa
o South America
________________________________________
Spotting Emerging Trends
Technological Advancements
Innovation in centrifugation systems and PRP kits has significantly improved platelet concentration efficiency and purity. Automated PRP preparation devices are reducing variability and enhancing reproducibility in clinical outcomes. Additionally, research into combination therapies (PRP with stem cells or hyaluronic acid) is expanding PRP’s potential across regenerative and cosmetic medicine.
Changing Consumer Preferences
Patients are increasingly seeking non-invasive, natural, and biologically derived treatments. PRP therapy fits this trend by using the body’s own healing components, reducing risk and recovery time. The growing popularity of aesthetic procedures like PRP facials (“vampire facials”) and hair restoration treatments has also driven consumer demand globally.
Regulatory Changes
Regulatory agencies such as the U.S. FDA and European Medicines Agency (EMA) are refining frameworks to ensure quality and safety in PRP preparation and use. These evolving guidelines are shaping product development, clinical trials, and commercialization strategies. In Asia-Pacific, supportive policies toward regenerative medicine are encouraging broader clinical adoption.
Growth Opportunities
The Platelet Rich Plasma Market is ripe with opportunities across diverse sectors:
• Sports and Orthopedic Medicine: Growing prevalence of musculoskeletal injuries and increased focus on faster recovery methods among athletes are driving clinical usage.
• Aesthetic Medicine: Expanding demand for PRP-based facial rejuvenation, acne scar reduction, and hair regrowth therapies.
• Wound and Chronic Ulcer Care: Enhanced efficacy of PRP in promoting angiogenesis and tissue repair presents vast potential in chronic wound management.
• Dental and Oral Surgery: PRP is increasingly utilized to improve bone regeneration and soft-tissue healing post-surgery.
• Research Collaborations: Joint research between biotechnology firms and healthcare providers is advancing PRP’s clinical applications.
• Emerging Markets: Expanding healthcare infrastructure in countries like India, China, and Brazil is fostering PRP adoption at affordable costs.
https://www.theinsightpartners.com/reports/platelet-rich-plasma-marketPlatelet Rich Plasma Market Report: Unlocking Growth Potential and Addressing Challenges
United States of America – [October 27, 2025] – The Insight Partners is proud to announce its latest report, “Platelet Rich Plasma Market: An In-Depth Analysis of the Global Industry Landscape.” The report provides a comprehensive view of the Platelet Rich Plasma (PRP) Market, describing the current scenario, emerging trends, and growth estimates during the forecast period 2023–2031.
Overview of the Platelet Rich Plasma Market
The Platelet Rich Plasma Market is witnessing dynamic expansion, fueled by rising applications in orthopedics, sports medicine, aesthetics, and wound healing. PRP therapy — derived from autologous blood concentrates enriched with platelets — is increasingly recognized for its regenerative potential. Its ability to accelerate tissue repair and reduce inflammation is transforming treatment protocols across both medical and cosmetic sectors.
This report provides in-depth insight into the market drivers such as technological innovations in PRP preparation systems, growing patient awareness, regulatory evolution, and an increasing preference for non-surgical treatment alternatives.
Market Segmentation
Segmentation Criteria:
1. By Type
o Pure Platelet Rich Plasma (P-PRP)
o Leukocyte-Rich Platelet Rich Plasma (L-PRP)
o Pure Platelet-Rich Fibrin (P-PRF)
o Leukocyte-Rich Platelet-Rich Fibrin (L-PRF)
2. By Origin
o Autologous PRP
o Allogeneic PRP
o Homologous PRP
3. By Application
o Orthopedics and Sports Medicine
o Dermatology and Aesthetics
o Neurosurgery
o Dentistry
o Wound Healing
o Others (Ophthalmology, Cardiology)
4. By End-User
o Hospitals and Clinics
o Research and Academic Institutions
o Ambulatory Surgical Centers
5. By Geography
o North America: United States, Canada, Mexico
o Europe: Germany, UK, France, Italy, Spain, Rest of Europe
o Asia-Pacific: China, Japan, India, South Korea, Australia, Rest of APAC
o Middle East & Africa
o South America
________________________________________
Spotting Emerging Trends
Technological Advancements
Innovation in centrifugation systems and PRP kits has significantly improved platelet concentration efficiency and purity. Automated PRP preparation devices are reducing variability and enhancing reproducibility in clinical outcomes. Additionally, research into combination therapies (PRP with stem cells or hyaluronic acid) is expanding PRP’s potential across regenerative and cosmetic medicine.
Changing Consumer Preferences
Patients are increasingly seeking non-invasive, natural, and biologically derived treatments. PRP therapy fits this trend by using the body’s own healing components, reducing risk and recovery time. The growing popularity of aesthetic procedures like PRP facials (“vampire facials”) and hair restoration treatments has also driven consumer demand globally.
Regulatory Changes
Regulatory agencies such as the U.S. FDA and European Medicines Agency (EMA) are refining frameworks to ensure quality and safety in PRP preparation and use. These evolving guidelines are shaping product development, clinical trials, and commercialization strategies. In Asia-Pacific, supportive policies toward regenerative medicine are encouraging broader clinical adoption.
Growth Opportunities
The Platelet Rich Plasma Market is ripe with opportunities across diverse sectors:
• Sports and Orthopedic Medicine: Growing prevalence of musculoskeletal injuries and increased focus on faster recovery methods among athletes are driving clinical usage.
• Aesthetic Medicine: Expanding demand for PRP-based facial rejuvenation, acne scar reduction, and hair regrowth therapies.
• Wound and Chronic Ulcer Care: Enhanced efficacy of PRP in promoting angiogenesis and tissue repair presents vast potential in chronic wound management.
• Dental and Oral Surgery: PRP is increasingly utilized to improve bone regeneration and soft-tissue healing post-surgery.
• Research Collaborations: Joint research between biotechnology firms and healthcare providers is advancing PRP’s clinical applications.
• Emerging Markets: Expanding healthcare infrastructure in countries like India, China, and Brazil is fostering PRP adoption at affordable costs.
https://www.theinsightpartners.com/reports/platelet-rich-plasma-market