Global Pediatric Clinical Trials Market to Reach USD 30.32 Billion by 2032-IMR Study
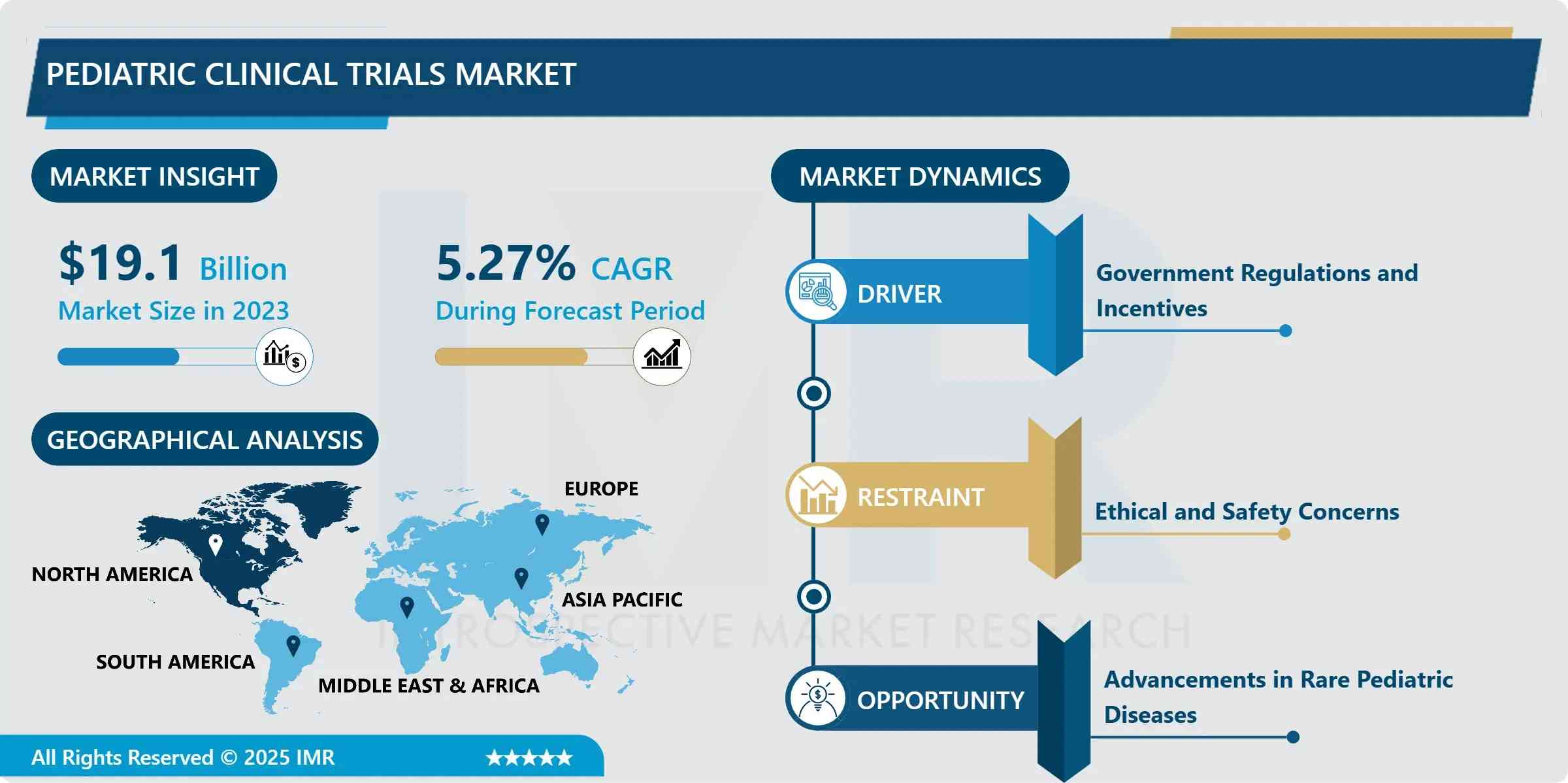
The Pediatric Clinical Trials Market is entering a pivotal growth phase, propelled by strengthened regulatory frameworks, rising incidence of chronic and rare pediatric diseases, and the accelerating integration of precision medicine in child-specific drug development. According to the latest analysis by Introspective Market Research, the market—valued at USD 19.10 Billion in 2023—is forecast to expand to USD 30.32 Billion by 2032, registering a compound annual growth rate (CAGR) of 5.27% from 2024 to 2032.
Pediatric clinical trials are specialized research protocols designed to evaluate the safety, efficacy, pharmacokinetics, and optimal dosing of investigational drugs, biologics, and devices in infants, children, and adolescents. Unlike adult trials, these studies must account for dynamic physiological maturation, age-stratified endpoints, ethical safeguards, and unique developmental vulnerabilities—making trial design significantly more complex yet critically essential. With over 70% of drugs prescribed to children historically lacking formal pediatric labeling, regulatory initiatives such as the U.S. Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA), alongside the EU’s Paediatric Regulation, are now mandating robust pediatric investigation plans (PIPs), dramatically expanding trial volume and investment.
Quick Insights: By the Numbers
- 2023 Market Size: USD 19.10 Billion
- 2032 Projected Value: USD 30.32 Billion
- CAGR (2024–2032): 5.27%
- Dominant Therapeutic Area: Oncology (accounting for largest share due to rising incidence of leukemias, CNS tumors, and neuroblastomas—and rapid adoption of molecularly targeted agents and immunotherapies)
- Largest Phase Segment: Preclinical (critical for modeling age-specific drug metabolism, toxicity thresholds, and developmental impact prior to human exposure)
- Top Regional Market: North America (over 40% value share in 2023), anchored by U.S. regulatory leadership, mature pediatric research networks, and extensive NIH/industry collaboration
- Key Players: Pfizer Inc. (USA), Roche (Switzerland), Novartis (Switzerland), Johnson & Johnson (USA), Sanofi (France), AstraZeneca (UK/Sweden), Merck & Co. (USA), GlaxoSmithKline (UK), AbbVie (USA), Bristol-Myers Squibb (USA), Bayer (Germany), Eli Lilly (USA)
Opportunity Spotlight: Can Decentralized Trials and AI-Driven Biomarker Discovery Overcome Recruitment Barriers and Accelerate Rare Disease Breakthroughs?
Two converging innovations are reshaping pediatric trial execution: decentralized clinical trial (DCT) models and genomic-guided patient stratification. Historically, low enrollment—driven by geographic concentration of pediatric centers, caregiver burden, and ethical hesitancy—has delayed timelines by 30–50%. Today, remote monitoring wearables, telehealth visits, and home-based sample collection are reducing site dependency, especially for chronic conditions like epilepsy and type 1 diabetes.
Simultaneously, next-generation sequencing (NGS) and liquid biopsy technologies are enabling ultra-precise patient selection—particularly in rare diseases and oncology. For instance, trials for NTRK-fusion tumors now enroll children across 12+ countries based on molecular signature rather than tumor origin. AI-powered platforms are also predicting optimal dosing windows using real-world growth and maturation data—reducing trial-and-error exposure.
Emerging markets in Asia Pacific and Latin America present high-growth potential, with India and Brazil establishing dedicated pediatric trial hubs supported by public–private partnerships and streamlined ethics review boards.
“Pediatric trials are no longer ‘add-ons’—they’re integral to lifecycle management and regulatory approval,” says Dr. Marcus Reynolds, Principal Consultant, Pediatric Drug Development & Regulatory Strategy at Introspective Market Research. “The field is shifting from reactive compliance to proactive therapeutic design. Companies that embed pediatric considerations from Phase I—using physiologically based pharmacokinetic (PBPK) modeling and adaptive trial designs—will secure faster approvals, premium reimbursement, and profound clinical impact. We’re now seeing first-in-child trials launch within 18 months of adult proof-of-concept, a timeline unthinkable a decade ago.”
Regional Leadership & Strategic Segmentation Breakdown
North America maintains market dominance, with the U.S. contributing ~75% of regional revenue. The FDA’s Office of Pediatric Therapeutics, coupled with networks like the Pediatric Trials Network (PTN) and Children’s Oncology Group (COG), enable rapid, multicenter recruitment and harmonized protocol execution. Canada and Mexico are scaling capacity, with Mexico emerging as a cost-efficient site for Latin American cohort expansion.
Europe follows closely, led by the UK, Germany, and France—where the EMA’s Paediatric Committee (PDCO) enforces strict PIP compliance. The EU’s Horizon Europe funding has prioritized rare disease trials, with over EUR 220M allocated to pediatric gene therapy studies in 2024 alone.
The Asia Pacific region is the fastest-growing segment (CAGR >6.1%), driven by China’s revised Drug Administration Law mandating pediatric assessments, Japan’s Sakigake designation for pediatric orphan drugs, and India’s Clinical Trials Registry expansion. Collaborations such as the Asia-Pacific Pediatric Clinical Trials Network (AP-PCTN) are standardizing GCP practices and boosting site readiness.
By Therapeutic Area, Oncology leads due to high unmet need and breakthrough therapies:
- Neurology is the fastest-growing segment—fueled by antisense oligonucleotides (e.g., for SMA, Dravet syndrome) and gene therapies for Rett and CDKL5 disorders.
- Infectious Diseases remain vital, particularly for novel vaccines (e.g., Lyme, RSV, universal flu) and antimicrobials targeting multidrug-resistant pathogens.
- Rare Genetic & Metabolic Disorders are seeing exponential pipeline growth, with >60% of current Phase II/III pediatric trials targeting orphan indications.
By Phase, Preclinical holds the largest revenue share—driven by high investment in juvenile animal toxicology, developmental and reproductive toxicity (DART) studies, and in silico modeling. However, Phase III is the fastest-growing segment as more molecules advance past safety gatekeepers.
Innovation Pipeline: Landmark Advances Reshaping the Standard of Care
Leading pharmaceutical and biotech firms are delivering transformative outcomes:
- Pfizer secured EU marketing authorization for PREVENAR 20® in July 2024—the first 20-valent pneumococcal conjugate vaccine approved for infants, children, and adolescents (6 weeks to <18 years), shown to reduce invasive pneumococcal disease by 84% in pivotal trials.
- Novartis launched the global SMArtCARE initiative, integrating newborn screening data with real-world treatment outcomes for Zolgensma®, enabling dynamic dose optimization and long-term safety surveillance across 28 countries.
- Roche advanced mosunetuzumab into pediatric Phase II for relapsed/refractory B-cell NHL—leveraging its T-cell engaging platform with reduced cytokine release syndrome (CRS) risk via step-up dosing.
- Sanofi and Sarepta Therapeutics jointly initiated the ENVISION-PEDS trial, evaluating an investigational exon-skipping therapy in ambulatory and non-ambulatory Duchenne muscular dystrophy (DMD) patients as young as 4 years—expanding eligibility beyond prior age restrictions.
Cost-Efficiency Strategies: Mitigating High Operational Burden Without Compromising Scientific Rigor
Pediatric trials cost 1.5–2.2× more per patient than adult studies due to specialized monitoring, caregiver support, and smaller cohort sizes. To optimize ROI, industry leaders are deploying:
- Master protocols with embedded substudies (e.g., basket/umbrella designs) to evaluate multiple agents or indications under one infrastructure—cutting setup time by 40%.
- Synthetic control arms powered by real-world data (RWD) to reduce placebo-group size, especially in ultra-rare diseases where randomization is ethically challenging.
- Modular consent platforms with animated, age-appropriate assent tools—improving comprehension and reducing dropout in adolescent populations.
- Regional CRO partnerships in Eastern Europe and Southeast Asia for cost-competitive site management (30–40% lower operational costs vs. Western hubs), without sacrificing data quality.
The strategic benefit? Earlier labeling expansions, priority review vouchers (PRVs), and extended market exclusivity—delivering both clinical and commercial upside.
About the Report
Pediatric Clinical Trials Market - Opportunities, Challenges & Strategic Forecast (2024-2032) delivers a 275+ page strategic assessment across 3 core dimensions: Phase (Preclinical, Phase I–IV), Therapeutic Area (Oncology, Neurology, Infectious Diseases, Rare Diseases, Cardiovascular, Respiratory, GI, Others), and Region (North America, Europe, APAC, MEA, South America). Includes deep-dive profiles of 12 key players, regulatory pathway mapping (FDA, EMA, PMDA, NMPA), trial database analysis (ClinicalTrials.gov, WHO ICTRP), and granular 15-year historical & forecast modeling (2017–2032).
Unlock Strategic Intelligence for Your Pediatric Pipeline
To receive a complimentary sample report, Visit:
https://introspectivemarketresearch.com/request/20141
Media Contact:
Sarah Kim
Director of Communications
Introspective Market Research
Email: info@introspectivemarketresearch.com
Phone: +91 91753-37569.
Website: https://introspectivemarketresearch.com
About Introspective Market Research
Introspective Market Research(IMR) is a globally recognized provider of high-integrity, data-driven intelligence for the biopharmaceutical, clinical development, and regulatory affairs sectors. Our team of MD/PhD-trained analysts, former FDA reviewers, and clinical operations experts delivers actionable insights that empower innovators—from emerging biotechs to multinational pharma—to de-risk development, accelerate approvals, and maximize patient impact. We combine proprietary trial databases, real-world evidence synthesis, and regulatory foresight to set the industry standard for depth, accuracy, and strategic relevance.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



